Success Story: Peter Caravan
In their words
The i3 was essential to the success of this project. The i3 provided technical support in writing, QA, and project management. The i3 provided access to expert consultants who gave invaluable guidance in the writing of the IND application and in responding to FDA questions.
The question
Peter wondered how to move a compound with promising pre-clinical results into human clinical trials. He had no direct experience with submitting an investigational new drug (IND) application to the Food and Drug Administration (FDA).
How we helped
The i3 brought in a regulatory consultant to advise Peter on writing, document handling, quality assurance, and submission. We also enlisted the help of a chemistry, manufacturing, controls (CMC) consultant to advise on the requirements for the CMC section.
The i3 managed the project, providing writing and editing support for the IND application, submission, and correspondence with the FDA. The i3 provided frameworks and writing/editing support for audited reports to support the regulatory submissions, and provided writing and regulatory support for the Institutional Review Board (IRB) application to use the probe at Massachusetts General Hospital.
The i3 also provided some financial support for a required toxicity study and finally, overall project management.
Collaboration results
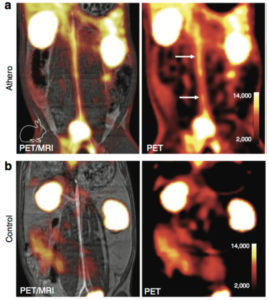
64Cu-FBP8, a positron emission tomography probe for detection of thrombus, received FDA approval to proceed to first in human studies.
Internally, this project helped strengthen the i3’s capacity to serve others in a number of ways:
• Development of future templates for INDs, and templates for ancillary documents such as study reports and study protocols.
• Establishment of a document management system and QA protocols.
• Creation of an internal style guide which includes document standards for I3-produced documents and serves as a how-to guide to collaborate on documents.
• Establishment of a relationship with the Martinos Center PET facility to transfer. probe technology to human production.
• Identification of useful contract research organizations for preclinical safety and analytical studies.
• Established a network of trusted external consultants.
• Implementation of project management tools for future translation of PET probes
• Established an infrastructure for physical submission of INDs and related documents to FDA.
Next big step
Continuing to work in collaboration with i3, Peter and his collaborators are using 64Cu-FBP8 to detect blood clots in patients with atrial fibrillation. The IND is being expanded to include patients with other pathologies.