Success Story: Umar Mahmood
In his words
Working with the i3 has been of tremendous value to our group. The regulatory expertise along with the i3’s experience in submitting INDs, as well as their understanding of the appropriate approaches for CMC has allow the researchers in our group to focus on developing the optimized imaging agent and developing an efficient clinical trial for testing it in breast cancer patients, and sharing the tasks to allow more rapid evaluation of our agent in patients. The time saved has allowed our science to move faster.
The problem
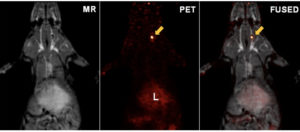
Dr. Mahmood’s lab identified a novel positron emission tomography (PET) probe that would help in the early detection of a common mechanism of therapy failure in breast cancer, i.e. this HER3 imaging probe could potentially identify which patients would gain from the addition of anti-HER3 therapy to their therapy regimens. This would allow those patients who are likely to benefit from the chance to receive such therapy, while saving patients who are unlikely to benefit from the side effects of these drugs.
After promising data in animal models, Dr. Mahmood wanted to translate this PET probe to human clinical trials. He had previously worked with the i3 in bringing the 68Ga-DOTATOC probe into human production at Massachusetts General Hospital. He realized that he would need the support of i3 to translate his new PET probe
How we helped
Dr. Mahmood was able to draw on the expertise and history of success of the i3 in a grant proposal to fund this development. He included members of the i3 team in his proposal and this, in part, led to success of the proposal. The i3 continues to provide regulatory and chemistry, manufacturing, controls (CMC) support to his investigational new drug (IND) application.
Collaboration results
Dr. Mahmood’s grant was funded.
Next big step
Work is now ongoing to translate the new HER3 peptide PET imaging probe.