Success Story: Peter Caravan and Pauline Desogere
In their words
The i3 provided the experience, expertise, and resources needed to execute this IND filing. The overall process was much more efficient than we could have managed on our own.
The problem
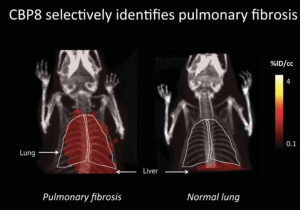
Pauline and Peter developed a new compound, 68Ga-CBP8: a positron emission tomography probe for detection of pulmonary fibrosis. The PET probe had promising pre-clinical results and they had a grant from The National Institutes of Health to move it into human clinical trials.
How we helped
The i3 managed the project, providing writing and editing support for the investigational new drug (IND) application. The i3 also provided frameworks and writing/editing support for audited reports to support the regulatory submissions, and regulatory support for the Institutional Review Board (IRB) application to use the probe at Massachusetts General Hospital.
Collaboration results
This project represented another successful example of the i3’s impact. The i3 played a major role in the execution of the IND application and resulted in further establishment of its protocols and processes for translating a novel PET tracer.
Next big step
The IND and IRB applications will be submitted in the fall of 2017.