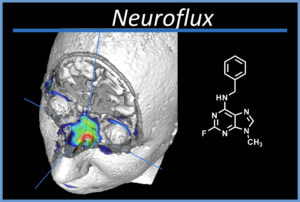
Success Story: Jacob Hooker
In their words
There are so many times in research that we find a gap in funding or knowledge. Neuroflux is a creative idea born from detailed preclinical work that needed to be tested in humans. A major barrier was funding for human-enabling toxicity studies. The support of i3 for funding and for advice through the IND preparation and submission process was simply incredible.
The problem
Dr. Hooker’s team needed guidance preparing for and writing an exploratory investigational new drug (eIND) application for the GV1-57 probe (later renamed Neuroflux). They also needed assistance completing studies in toxicology, stability, and radiation dosimetry.
How we helped
The i3 provided a document management solution to Dr. Hooker’s team, providing templates for reports that incorporated auditable version control and stored the documents in a centralized server. i3 team members served in project management and editorial roles. We also played a consulting role on regulatory questions that arose throughout the process. The i3 also provided matching funds to enable a pivotal in vivo toxicity study.
Collaboration results
The application was submitted successfully and Dr. Hooker’s team received FDA approval to proceed.
Next big step
Neuroflux has been used for imaging healthy volunteers and people with anosmia (loss of smell) at MGH.