Success Story: Jacob Hooker
In his words
Without the help of the i3, we would not have had the confidence to move so quickly from our preclinical work to human research. A first submission to the FDA can be daunting and having access to an experienced regulatory consultant was amazing. In fact, our relationship to the consultant has persisted and this has expanded our network and made stronger connections to the pharma industry than you would imagine from face value of the i3 contribution. Connecting resources is a massive benefit that the i3 brings to MGH!
The problem
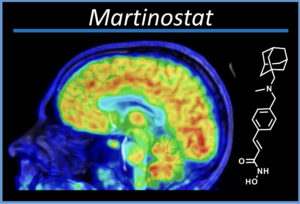
Dr. Hooker submitted an exploratory investigational new drug (eIND) application to the Food and Drug Administration (FDA) for Martinostat. It was his first submission and he wanted to make sure the submission was appropriately formatted and contained all the crucial information. The FDA responded with a number of questions.
How we helped
The i3 identified an external regulatory consultant to work with Dr. Hooker to review the initial application submission and respond to the FDA’s questions.
Collaboration results
The FDA was satisfied, the eIND was allowed to proceed, and Dr. Hooker was able to commence studies in humans.
Next big step
Martinostat PET has been successfully used in a number of human research studies at Massachusetts General Hospital including patient population of schizophrenia, Alzheimer’s disease, ALS, Huntington’s disease (among others) and has recently been introduced at least two other institutions.